Stabilizing Oxygen Redox in Na-Ion Battery Cathode Through Spin Singlet State
April 26, 2024
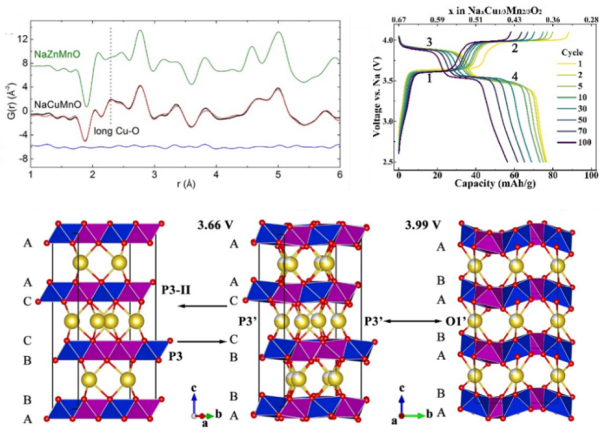
(top left) Neutron pair distribution function showing the Jahn-Teller distortion associated with Cu2+. (top right) Charge and discharge profiles of P3-type Na2/3Cu1/3Mn2/3O2. (bottom) The reversible structure changes during charge/discharge.
Scientific Achievement
A non-hysteresis, long-term stable lattice oxygen redox reaction is realized in the newly synthesized P3-type Na2/3Cu1/3Mn2/3O2. Spin singlet is found to be the major mechanism that stabilizes the active oxygen redox reaction.
Significance and Impact
This discovery offers an alternative charge compensation mechanism that stabilizes the active oxygen redox reaction. Paving the route to discovery new types of low-cost Na-ion battery cathodes.
Research Details
- P3-type Na2/3Cu1/3Mn2/3O2 structure was determined by combining high resolution neutron diffraction, X-ray diffraction, neutron pair distribution function and transmission electron microscope.
- In-situ hard X-ray absorption and ex-situ soft X-ray absorption spectrum, along with density function theory calculations, reveal two distinct charge compensation mechanisms at different voltage plateaus.
Stabilizing lattice oxygen redox in layered sodium transition metal oxide through spin singlet state
Nature Communications 14, 7665 (2023).
DOI: https://doi.org/10.1038/s41467-023-43031-6