Mapping Hydrogen Atoms in SARS-CoV-2 Main Protease
Scientific Achievement
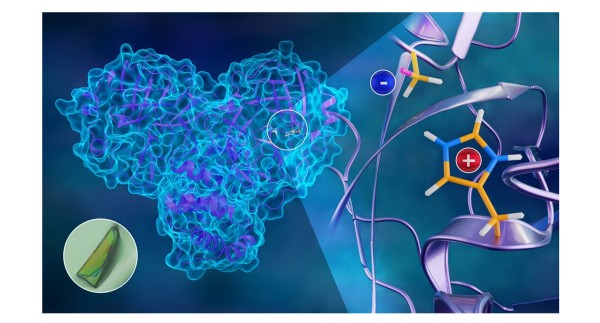
The positions of hydrogen atoms have been located enabling a full determination of the hydrogen bonding and protonation states of the main protease (3CL Mpro) of SARS-CoV-2.
Significance and Impact
Inhibiting 3CL Mpro prevents the virus from replicating, and understanding its structure is an important step in designing new drugs to combat SARS-CoV-2.
Research Details
- Single crystals of 3CL Mpro were grown, enabling diffraction studies.
- The full structure was determined by jointly refining x-ray and neutron diffraction data.
- The neutron data allowed for visualization of the protonation states and hydrogen bonding.
“Unusual zwitterionic catalytic site of SARS-CoV-2 main protease revealed by neutron crystallography”
Daniel Kneller, Gwyndalyn Phillips, Kevin Weiss, Swati Pant, Qiu Zhang, Hugh O’Neill Leighton Coates, and Andrey Kovalevsky,
Journal of Biological Chemistry, (2020).
DOI: https://www.jbc.org/content/early/2020/10/15/jbc.AC120.016154