Nanoparticle Supercrystal Assembly from Solution
Scientific Achievement
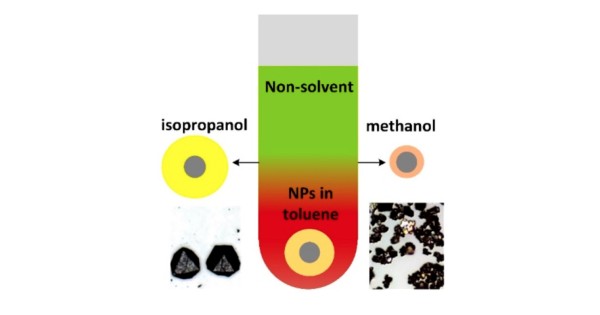
Nonsolvent alcohols used to form PbS nanoparticle supercrystals (SCs) determine assembly kinetics and control the ligand shell size, leading to large SC structures with large lattice constant when shells are thick (isopropanol) and small SCs with small lattice constant when shells are thin (methanol).
Significance and Impact
The results improve the understanding of assembly of purified NP SCs from solution, aiding the development of new materials with diverse applications.
Research Details
- The influence of alcohols on ligand conformation was studied by controlling contrast in small-angle neutron scattering (SANS).
- Crystal packing symmetry was determined in situ by SAXS and correlated with nanoparticle ligand conformation measured by SANS.
“Revealing the Effects of the Non-solvent on the Ligand Shell of Nanoparticles and Their Crystallization,”
Byeongdu Lee, Kenneth Littrell, Yuchen Sha, and Elena V. Shevchenko,
Journal of the American Chemical Society, 141, 16651−16662 (2019). DOI: https://doi.org/10.1021/jacs.9b06010