One of the biggest factors affecting consumer adoption of electric vehicles (EVs) is the amount of time required to recharge the vehicles—usually powered by lithium-ion batteries. It can take up to a few hours or overnight to fully recharge EVs, depending on the charging method and amount of charge remaining in the battery. This forces drivers to either limit travel away from their home chargers or to locate and wait at public charging stations during longer trips.
Why does it take so long to fully charge a battery, even those used to power smaller devices, such as mobile phones and laptops? The primary reason is that devices and their chargers are designed so the rechargeable lithium-ion batteries charge only at slower, controlled rates. This is a safety feature to help prevent fires, and even explosions, due to tiny, rigid tree-like structures, called dendrites, that can grow inside a lithium battery during fast charging and induce short-circuits inside the battery.
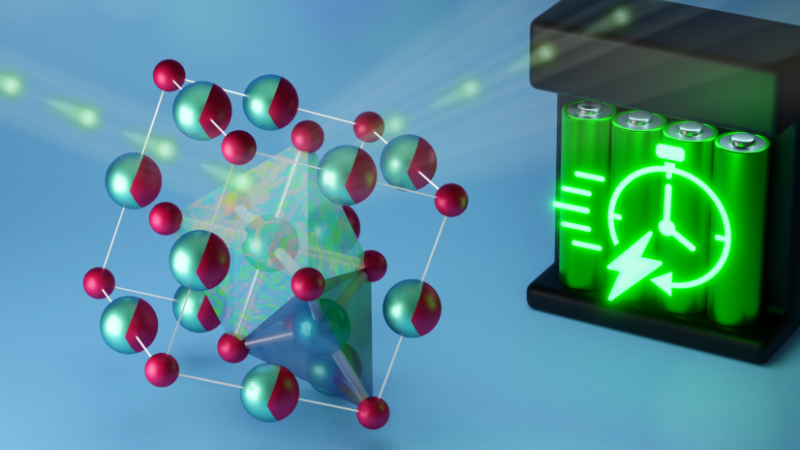
To address the need for a more practical lithium-ion battery, researchers from the University of California San Diego (UC San Diego) worked with scientists at Oak Ridge National Laboratory (ORNL) to conduct neutron scattering experiments on a new type of material that could be used to make safer, faster-charging batteries. The researchers produced samples of lithium vanadium oxide (Li3V2O5), a “disordered rock salt” similar to table salt but with a certain degree of randomness in the arrangement of its atoms. The samples were placed in a powerful neutron beam that enabled observing the activity of ions inside the material after a voltage was applied.
The results of the research were published in the journal Nature in a paper titled “A disordered rock salt anode for fast-charging lithium-ion batteries.”
“The two most common materials used to make lithium-ion battery anodes are graphite, which can deliver high energy density but has caused fires in some situations, and lithium titanate, which can charge rapidly and is much less likely to cause fires but has lower energy storage capacity,” said Haodong Liu, a research scientist in professor Ping Liu’s lab at UC San Diego and first author of the paper. “The disordered rock salt material we developed combines the desired properties of both—it is safer, faster-charging, and has a higher energy density.”
The material demonstrated desirable qualities for many battery applications, such as EVs and power tools, including how fast the energy can be stored and discharged for use.
During testing, the rock salt anode material was able to deliver more than 40 percent of its energy capacity in just 20 seconds. The rapid charging and discharging appear possible because the rock salt material can cycle two lithium ions in and out of vacant sites within its crystal structure.
“Using neutron diffraction techniques at ORNL enabled us to understand how the ions behave when we applied voltage to the materials,” said Liu. “Neutrons can easily track lithium ions and oxygen atoms inside the rock salt anode, and using the VULCAN instrument at ORNL’s Spallation Neutron Source (SNS) provided the high neutron flux and resolution we needed.”
VULCAN is designed for neutron studies of deformation, phase changes, residual stress, texture, and microstructures in engineering materials. Load frames, furnaces, battery cyclers, and other auxiliary equipment for in situ experiments (where a material is studied as is), and steady-state (continuous) or time-resolved (a series of “snapshots”) measurements are integrated with the instrument.
“VULCAN is the world’s top neutron scattering instrument for studying engineered materials,” said Ke An, an ORNL neutron scattering scientist. “Its open design permits large samples and even functioning mechanical devices, such as running combustion engines, to be tested and to observe their internal properties. The instrument has provided critical scientific information for energy storage research during battery materials synthesis as well as their behaviors in working batteries.”
The researchers showed that the rock salt anode can be cycled over 10,000 times with negligible capacity decay. Such durability would be important for consumer applications.
“This research is part of a long-term collaboration between our research group and ORNL, which has resulted in more than 20 peer-reviewed journal papers,” said Liu. “The staff at ORNL’s Neutron Sciences Directorate has worked closely with me and my colleagues to help us learn about the capabilities of neutrons, and they have taught us how to use the instruments to conduct experiments and interpret the data.”
Researchers also performed high-resolution microscopic studies to resolve the structural changes at the University of California Irvine and DOE’s Brookhaven National Laboratory.
Once these experiments and those at ORNL were completed, scientists at Argonne National Laboratory, as well as scientists from DOE’s Lawrence Berkeley National Laboratory, conducted x-ray diffraction and x-ray absorption studies to reveal the crystal structural change and the charge compensation mechanisms of the material during charging and discharging.
Like most users, UC San Diego retained its rights to the data and any intellectual property produced during the experiments. In order to commercialize their discovery, the university subsequently worked with its researchers to form a company called Tyfast, which plans to first target the electric bus and power tool markets.
The neutron scattering research was supported by the DOE Office of Science.
SNS is a DOE Office of Science user facility. ORNL is managed by UT-Battelle LLC for DOE’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science is working to address some of the most pressing challenges of our time. For more information, visit energy.gov/science. –by Paul Boisvert