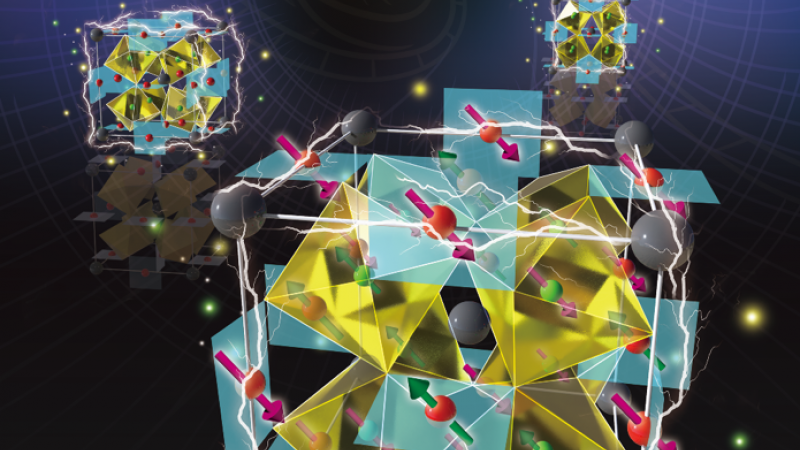
Materials used in electronic devices are typically chosen because they possess either special magnetic or special electrical properties. However, an international team of researchers using neutron scattering recently identified a rare material that has both.
In their paper published in Advanced Materials, the team, including researchers from the Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL), illustrates how this unique marriage is achieved in the multiferroic material BiMn3Cr4O12. Many materials are known for just one characteristic magnetic or electrical property, or for having the ability to change shape, but multiferroics contain some combination of these attributes.
Multiferroics are typically divided into two distinct categories: conventional (type-1) and unconventional (type-2). Conventional multiferroics are predominantly controlled by electricity and exhibit weak interactions with magnetism. Conversely, unconventional multiferroics are driven by magnetism and exhibit strong electrical interactions.
“We have found an interesting example of joint multiferroicity, meaning that both conventional and unconventional multiferroicity develop one after the other in the same material,” said ORNL researcher Huibo Cao.
One reason multiferroics are so desirable is that their dual characteristics can be controlled in combination with each other, providing, for example, electrically controlled magnetism or magnetically controlled electrical properties. Researchers say a better understanding of how these multifunctional materials behave could lead to significant advances in information storage and power performance in new devices.
For example, materials with the optimized combination of both multiferroic mechanisms could be used as efficient switches, magnetic field sensors, and memory devices.
“With this material, we see the potential to reach beyond the typical scope of multiferroic applications and make a significant impact on a variety of practical projects,” Cao said.
These insights could also serve as a foundation to help researchers develop similar materials containing this blend of properties.
“The existence of this rare material and the ability to find others like it provide a new range of exciting possibilities for future research and development,” said ORNL researcher Stuart Calder.
Neutrons are the most suitable probe to study the magnetism of these materials and provide a distinction between the different types of multiferroic behavior. Because neutrons have no charge, they can easily examine crystal structure behavior in complex sample environments such as pressure cells. At the same time, they have spin and the ability to behave like magnets, making them ideal for studying magnetism.
By exposing a sample to varying temperatures, magnetic/electric fields, and pressures, the researchers can observe how the atomic structure and magnetic properties respond to environmental factors and to each other, which could further guide the design of new materials.
The team performed neutron scattering measurements at ORNL’s High Flux Isotope Reactor (HFIR), a DOE Office of Science User Facility. Using the Neutron Powder Diffractometer instrument, HFIR beamline HB-2A, they determined how the material’s magnetic structures correlate to its ferroelectric polarization, which is the slight separation between the centers of positive and negative charge in the atomic units making up the crystal structure.
“With neutrons, we can see how these magnetic structures are ordered to better understand the different types of multiferroics,” Calder said. “We are beginning to solve some of the mysteries that surround these materials.”
The research is supported by DOE’s Office of Science. HFIR is a DOE Office of Science User Facility. UT-Battelle manages ORNL for the DOE’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit http://science.energy.gov/.—by Elizabeth Rosenthal