Although they don’t currently have as much conductivity, solid-state electrolytes designed for lithium-ion batteries (LIBs) are emerging as a safer alternative to their more prevalent—sometimes flammable—liquid-electrolyte counterparts.
However, a new study conducted at Oak Ridge National Laboratory’s Spallation Neutron Source (SNS), a Department of Energy Office of Science user facility, has revealed promising results that could drastically boost the performance of solid-state electrolytes, and could potentially lead to a safer, even more efficient battery.
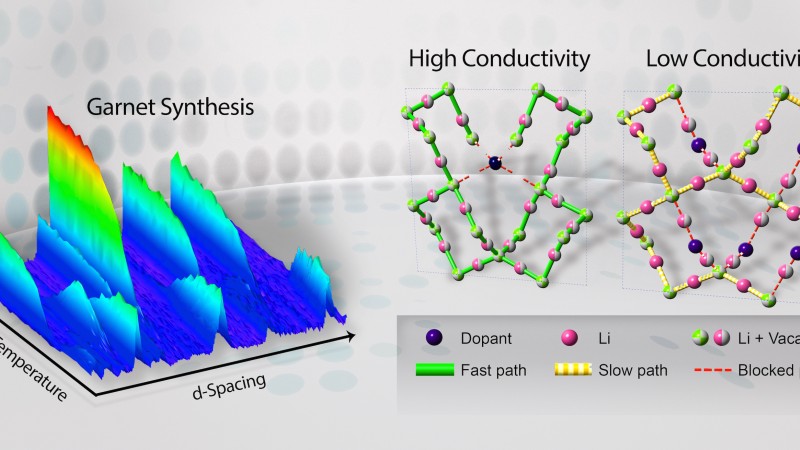
Using neutron diffraction techniques via the VULCAN instrument, SNS beam line 7, lead instrument scientist Ke An and his team recently concluded an in-depth study probing the entire structure evolution of doped garnet-type electrolytes during the synthesis process to unravel the mechanism that boosts the lithium-ionic conductivity. Their findings were recently published in the journals Chemistry of Materials and the Journal of Materials Chemistry A.
“The question we want to answer is how can we correlate the material’s structure with its performance,” An said. “Finding an answer to this will be very useful to the materials community, particularly in the field of electrochemical devices like batteries.”
The problem with liquid electrolytes, says An, is that while they can produce high levels of conductivity—which is good—in some cases, they become flammable under high voltages or high temperatures, causing the battery to “explode”—which is obviously very bad.
In general, solid electrolyte-based LIBs consist of two electrodes, a positive and a negative, and an electrolyte in the middle, forming the battery’s core, which facilitates the movement of ions traveling back and forth between the electrodes. In order to achieve a desired level of conductivity in the electrolyte, ions require vacancies in the crystal structure, or tunnels for the ions to “hop” to and from—kind of like connecting the dots.
Lithium lanthanum zirconates, or materials based on Li7La3Zr2O12 with a garnet structure, are favorable for application as electrolytes because they promote fast lithium transport. However, explained An, synthesized garnets often develop unwanted low-conductivity secondary phases, which in some cases can be detrimental to electrolytic performance. Essentially what that means is that useful vacancies for ions to “hop” don’t always develop where designers want them to.