Article courtesy of the Max Planck Institute for Intelligent Systems
Written by Linda Behringer
Hydrogen (H2) is currently discussed as an ideal energy carrier in a world requiring renewable energies. Hydrogen has the highest gravimetric energy density of all chemical fuels (141 MJ/kg), which is three times higher than gasoline (46 MJ/kg). However, its low volumetric density restricts its widespread use in transportation applications—as current storage options require a lot of space.
At ambient temperature, hydrogen is a gas, and one kilogram of hydrogen occupies a volume of 12000 liters (12 cubic meters). In fuel-cell vehicles, hydrogen is stored under a very high pressure of 700 times the atmospheric pressure, which reduces the volume to 25 liters per kilogram of H2. Liquid hydrogen shows a higher density resulting in 14 liters per kilogram, but it requires extremely low temperatures since the boiling point of hydrogen is minus 253 °C.
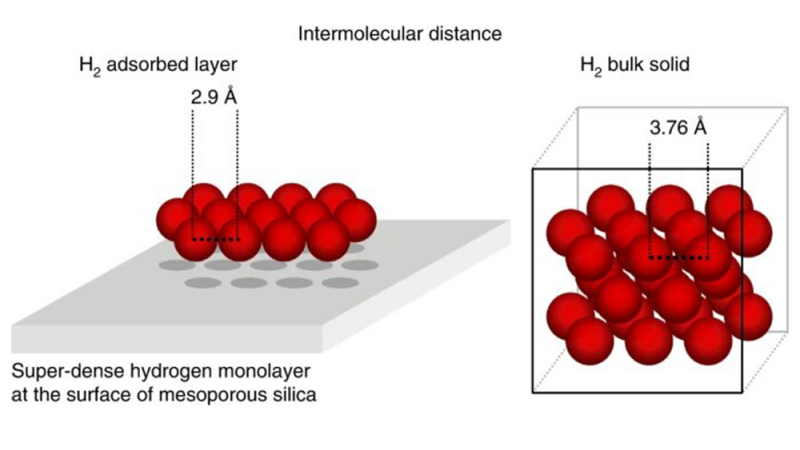
Now a team of scientists from the Max Planck Institute for Intelligent Systems, the Technische Universität Dresden, the Friedrich-Alexander-Universität Erlangen-Nürnberg, and the Department of Energy’s (DOE) Oak Ridge National Laboratory (ORNL) has demonstrated that hydrogen condenses on a surface at a very low temperature near the H2 boiling point, forming a super-dense monolayer exceeding the density of liquid hydrogen by a factor of almost three, which reduces the volume to only 5 liters per kilogram H2.
The surprising result was that twice as many H2 molecules than atoms of the noble gas argon covered the surface, even though both have nearly the same size. To double the number of molecules per area, H2 molecules squeeze closely together, forming a super-dense layer.
Published in Nature Chemistry, the study by R. Balderas-Xicohténcatl et al. involved high-resolution cryo-adsorption experiments on highly-ordered mesoporous silica exhibiting well-defined pore and surface characteristics to determine the number of molecules condensed on the material’s surface.
Inelastic neutron scattering is an ideal tool to follow the formation of this two-dimensional hydrogen layer. For the first time, the existence of this super-dense hydrogen was confirmed in situ. This direct observation was only possible using the high-resolution neutron vibrational spectrometer VISION at ORNL’s Spallation Neutron Source (SNS), which features an inelastic count rate more than 100 times greater than any similar available spectrometer.
Theoretical studies confirm the experimental observations of the unusually high hydrogen density in the adsorbed layer. The attractive forces at the surface were stronger than the repulsion between two hydrogen molecules resulting in a super-dense hydrogen packing on the mesoporous silica surface. The super-high density is a consequence of the high compressibility of hydrogen, which doesn’t have core electrons.
The formation of the super-dense hydrogen layer at low temperatures near the boiling point is of fundamental interest. It should be considered for quantitative analysis of H2 adsorption isotherms at 20 K. It may also open new possibilities for enhancing the volumetric capacity of cryogenic hydrogen storage systems for many applications in a coming hydrogen economy.
+Click here to read the original article
Original publication:
Rafael Balderas-Xicohténcatl, Hung-Hsuan Lin, Christian Lurz, Luke Daemen, Yongqiang Cheng, Katie Cychosz Struckhoff, Remy Guillet-Nicolas, Gisela Schütz, Thomas Heine, Anibal J. Ramirez-Cuesta, Matthias Thommes, Michael Hirscher. Formation of a super-dense hydrogen monolayer on mesoporous silica. Nature Chemistry. https://doi.org/10.1038/s41557-022-01019-7
SNS is a DOE Office of Science user facility. UT-Battelle manages ORNL for the DOE Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit www.energy.gov/science.