Nitrogen dioxide and sulfur dioxide (NO2 and SO2) are toxic gases harmful to the environment and human health. Once they enter the atmosphere, they can travel hundreds of miles, polluting the air and causing acid rain which in turn damages buildings, trees, and crops. Exposure to the toxic gases can also lead to respiratory infections, asthma, and chronic lung disease.
For those reasons, the so-called acid gases are high on the list of pollutants targeted by the Clean Air Act, which requires the Environmental Protection Agency to regulate and set limits on NO2 and SO2 emissions with the goal of improving air quality and preventing widespread illnesses.
Scientists are developing materials that can detect and trap acid gases, an effort among some of the leading innovative strategies to mitigate air pollution and combat climate change. The approach consists of various technological solutions designed to filter the air by capturing or trapping toxic gases from emissions. In some cases, captured molecules can also be stored and reused—carbon dioxide, for example, can be reused in certain applications to promote photosynthesis and plant growth.
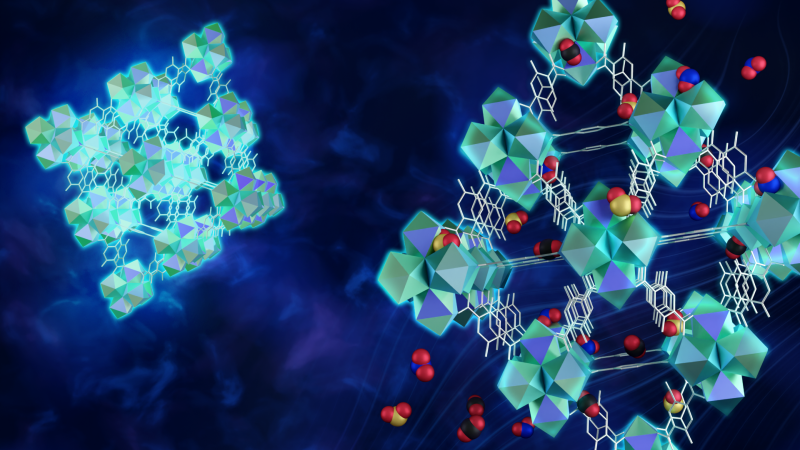
Materials called metal organic frameworks, or MOFs, could take acid-gas sequestration to the next level, making it a more viable, practical approach to improving air quality on a global scale. MOFs are essentially a microscopic matrix of metal atoms attached to each other by organic molecules that form a repeating pattern of tiny, interconnected metal cages. They act like a sponge that can adhere, or soak up, molecules to its surface. In fact, MOFs are so highly porous that the amount that would fit inside someone’s pocket, if stretched out, would cover the surface of an entire football field.
In a recent study published in the journal ACS Applied Materials & Interfaces, researchers searching for candidate materials to remediate NO2 and SO2 investigated a series of MOFs that can be made from the entire family of rare-earth metals. They used computer simulations and a combination of neutron and x-ray scattering experiments to help them determine the optimal conditions for synthesizing the materials. In the process, they also uncovered important details about an interesting defect that forms in the MOFs that they say could be useful in building devices for capturing emissions or sensing dangerous levels of toxic gases.
“Metal organic frameworks are really novel in their flexibility, their chemistry, and how you can tailor their structure. If you swap out organic molecules, you can tune the structure to target different gases,” said Sandia National Laboratory’s Susan Henkelis, the study’s lead author. “Acid gases typically come from combustion processes, so this research could be useful in developing devices to help limit emissions from large-scale industrial facilities like oil refineries and fossil fuel-based power plants.”
The team includes researchers from the Department of Energy’s (DOE) Sandia and Oak Ridge national laboratories (ORNL) and the University of Tennessee, Knoxville (UTK). The researchers are part of the Center for Understanding and Control of Acid Gas-Induced Evolution of Materials, or UNCAGE-ME, a program developed specifically to understand the interactions between acid gases and solid materials. UNCAGE-ME is part of a broader research effort supported by DOE’s Energy Frontier Research Center (EFRC) program, which brings together the research capabilities of universities and national laboratories to provide atomic-scale insights into tackling some of the world’s biggest energy challenges that can only be achieved through large collaborations.
“The fundamental science objective of this work was aimed at understanding how the chemistry and the synthesis process creates these defects, because we want to know how the defects can be controlled and what their affect is on adsorption of acid gases,” said Peter Metz, a postdoctoral researcher at UTK who worked in Neutron Sciences at ORNL during the time of the study. “To do that, we need to understand how the atomic bonds in the MOFs form and how the atoms are arranged.”
Ideally, the cages inside each synthesized MOF form a cube. Each corner contains a cluster of six rare-earth metal ions with another cluster in the center of the cube. Each pair of metal ions in the cluster connects to another pair in another cluster by a single link, or linker molecule.
But sometimes a defect occurs, especially in MOFs made of europium ions, where the linker kinks and exposes the rare-earth ion, which increases the likelihood a pollutant molecule will become trapped within the structure.
To find out why this happens, the researchers used a combination of neutron and x-ray scattering experiments to map the materials’ atomic structures.
They used x-rays to find the heavy metal elements, which provided an outline of the overall structure. And, to better understand how the organic molecules are arranged, they bombarded the materials with neutrons using the POWGEN instrument at ORNL’s Spallation Neutron Source (SNS), which helped them track the positions of the hydrogen, carbon, and oxygen atoms that form the molecular bonds between the metal ion clusters.
From the experiments, the team was able to determine that the materials with the defects actually formed more rapidly than their defect-free counterparts. They also discovered the defects could be intentionally induced by adjusting the temperatures and the time it takes to grow the crystalline materials.
The team then used the structural data obtained from the experiments to run computer simulations to see how each of the materials—with and without the defects—interacted with the toxic gases NO2 and SO2.
“While these new insights are on the basic research side of things, they could have a big impact down the road,” said Sandia’s Tina Nenoff, the study’s corresponding author. “We learned new information about how these materials form, which we can use to control and design MOFs with more specificity. And furthermore, we developed a comprehensive approach to evaluating large series of MOFs, which will help expedite the pace of finding new candidate materials and developing them in useful technologies.”
In addition to Henkelis, Nenoff, and Metz, the paper’s co-authors include Sandia’s Dayton J. Vogel, Nichole R. Valdez, Mark A. Rodriguez, David X. Rademacher, Stephen J. Percival, Jessica M. Rimsza, ORNL’s Stephen Purdy, and UTK’s Katharine Page. The POWGEN neutron measurements were performed by ORNL’s Cheng Li.
SNS is a DOE Office of Science user facility. UT-Battelle LLC manages ORNL for the DOE Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit https://www.energy.gov/science/office-science.—by Jeremy Rumsey