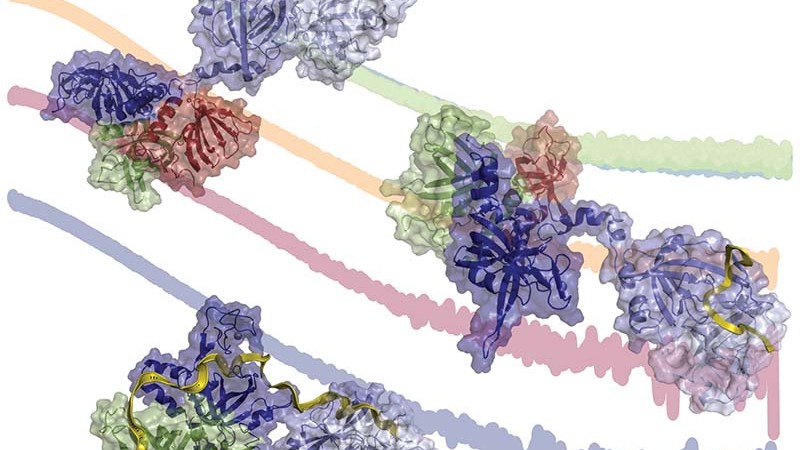
We now know that many serious diseases have genetic links that a geneticist can find by reading an individual’s genome─the DNA double helix where our organism’s hereditary information is encoded. Researchers know too that a particular protein protects our DNA, which is vulnerable to entanglement when its information is read and to attack from enzymes that damage the strands, making the code indecipherable.
This precious protein is called replication protein A. If DNA were the words in a book, then the RPA protein would make sure the pages were kept in place when the book was open and that the reader read the words in correct order. As the primary single-stranded DNA binding protein in humans, RPA is absolutely essential to virtually all processes that maintain and propagate our genomes.
“To be read, the DNA needs to be unwound from its familiar, double-stranded helical state into separate strands,” explains Walter Chazin of the Departments of Biochemistry and Chemistry, and director of the Center for Structural Biology, at Vanderbilt University. “But ssDNA has a strong tendency to become tangled and is also readily cut up by numerous nuclease enzymes. Every organism has evolved ssDNA binding proteins to protect the ssDNA and keep it untangled.”
RPA functions by serving as a platform for the many highly dynamic, multi-protein machines that perform these essential processes. The protein not only manages access to the ssDNA but also orchestrates the coming and going of the other proteins that make up the entire DNA processing machine.
Despite its central importance, little is known about how RPA molecules perform these complex roles. In a recent study, Chazin and his collaborators used the CG-3 Bio-SANS instrument at the High Flux Isotope Reactor at Oak Ridge National Laboratory as part of a multi-pronged approach to study this key ssDNA binding protein. In the course of the work, they developed a new method for studying these proteins that can be applied to other biosystems. Their work is being published as a featured article in Oxford University’s Nucleic Acids Research Journal.
With the help of neutron scattering at HFIR, x-ray scattering at the Advanced Light Source at Lawrence Berkeley National Laboratory, computational resources, and the resources of the Vanderbilt Center in Molecular Toxicology and the Vanderbilt Ingram Cancer Center, the researchers developed a fundamentally new approach to examining the multi-stage process by which RPA engages DNA.
“The results alter the long-standing views of RPA,” says Chazin. “They contrast with previous models that had proposed that RPA initially binds ssDNA in a condensed state and becomes more extended as it fully engages the substrate. The consensus view that RPA engages ssDNA in initial, intermediate, and final stages conflicts with what we found,” says Chazin. “Our work reveals that RPA undergoes two (not three) transitions as it binds ssDNA, with no evidence for a discrete intermediate state.”
The data provide a new framework for understanding both how RPA works and how it is able to stimulate DNA processing machines. Further, it provides new insights into how one DNA processing pathway can be selected over others.
The HFIR Bio-SANS instrument provided the core results for the study, Chazin says. The small-angle neutron scattering experiments confirmed that their interpretation of the data was correct. ORNL’s Deuteration Laboratory also supplied essential equipment to prepare the researchers’ samples on site.
The work both answers fundamental science questions about the protein RPA and demonstrates an important new approach for studying many other systems of high biological importance, Chazin says. “These and related studies also provide the critical foundation for translating our research to clinical applications. We are already involved in trying to evaluate the potential therapeutic value of inhibiting RPA with small molecules, as an adjuvant to cancer chemotherapies that work by damaging the DNA of cancer cells.” Their next step is to use the new strategy they have developed to characterize how RPA configures itself when it performs different functions with human DNA and when it repairs damaged DNA with the critical repair factor XPA (another protein involved in DNA repair).
Working with Chazin and Chris A. Brosey of Vanderbilt University are Chunli Yan and Ivaylo Ivanov of Georgia State University; Susan E. Tsutakawa, Robert P. Rambo, and John A. Tainer of LBNL; and William T. Heller of ORNL.
Funding for the neutron scattering work comes from the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy, and the DOE Office of Biological and Environmental Research. X-ray scattering at the LBNL Advanced Light Source was funded by the DOE Integrated Diffraction Analysis Technologies program and by a grant from the National Institutes of Health. Further funding came from NIH, Georgia State University, the National Science Foundation, and the DOE Office of Science National Energy Research Scientific Computing Center.
Published Work:
C. A. Brosey, C. Y., S. E. Tsutakawa, W. T. Heller, R. P. Rambo, J. A. Tainer, I. Ivanov, and W. J. Chazin, “A new structural framework for integrating replication protein A into DNA processing machinery,” Nucleic Acids Research 41 (2013), DOI: 10.1093/nar/gks1332.