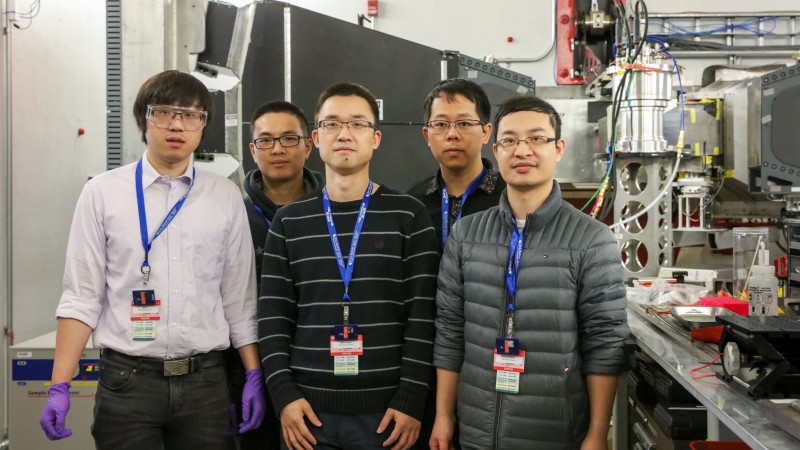
Finding new, more efficient ways to produce power is a critical mission for the Department of Energy (DOE), and developing more advanced materials is often the key to achieving success.
Researchers from West Virginia University (WVU) are using neutron scattering at the DOE’s Oak Ridge National Laboratory (ORNL) to study novel materials called high-entropy oxides, or HEOs. Their goal is to collect insights into how the atoms in the HEOs bind together and whether the materials can be used to develop useful applications to improve power plant operations.
Efficiency affects overall costs for fuel and plant environmental performance. Currently, they are developing HEOs for several applications including a high-temperature gas sensor that will be used to detect carbon monoxide in the flue gas of a coal-fired power plant to allow operators to monitor the plant’s efficiency. A similar sensor is being tested at the Longview Power plant in Maidsville, WV, near the WVU main campus.
“HEOs are materials that consist of four or more metal oxides mixed together in a certain ratio or proportion to form a homogeneous structure,” said WVU materials scientist Wei Li, who led the five-person team in conducting the ORNL neutron scattering experiments. “We’re using neutrons to see if the materials mix evenly into a single oxide phase or whether they separate into multiple phases, in which case we would need to adjust the ratios of the material’s elements, as well as the manufacturing conditions, to ensure the materials form homogeneously the way we want them to.”
Research into HEOs is increasing because of their advanced properties such as high resistance to heat and corrosion, as well as their multifunctionality, or potential for dielectric, electrochemical, and catalytic applications. The idea is the more metal oxides that can be successfully mixed together, the more beneficial properties the material will have.
Most HEOs are synthesized by heating mixtures of metal oxide powders at high temperatures, then cooling the resulting material into a single solid phase. However, says Li, it’s unclear how uniform single-phase HEOs form from the nonuniform, or inhomogeneous, mixtures of raw materials.
Fewer footprints, better batteries
The team is performing a series of neutron scattering experiments to study two types of HEOs. The first material is made of magnesium, cobalt, nickel, copper, and zinc oxides—atomically arranged in a cube-shaped rock-salt structure, like sodium chloride. The second material the team is studying is a perovskite, made from rare-earth and transition metals (plus oxygen).
To lower the carbon footprint, the team intends to develop the first type of HEO material into a gas sensor that can be mounted high inside a power plant’s exhaust stack, where temperatures range around 1,800°F (about 980°C).
“The sensors will be placed in hard-to-reach areas with harsh conditions. Achieving a single phase is important for the stability of the material and its sensitivity to detect carbon monoxide that we want to prevent from reaching the atmosphere,” said Li.
What’s more, the raw form of the material used to make the gas sensor can also be used to make components for advanced lithium batteries, just by adding lithium oxide to the list of the raw ingredients ((MgCoNiCuZn)1- xLixO1-δ).
Li says the lithium-ion batteries currently employed in certain power plants to store excess energy use graphite-based electrodes, which offer good stability but have limited storage capacity. Li is working to upgrade to more robust lithium batteries, but finding high-capacity electrodes with stability comparable to that of graphite poses a challenge. With that in mind, the team aims to use a high-entropy metal oxide to develop an improved electrode for a lithium-ion battery that offers high lithium conduction properties as well as exceptional stability for long-term charging and discharging cycles.
Perovskite’s potential
With the perovskite, the team wants to design a catalyst to be used in the development of a fuel cell that can provide an alternative means of generating large amounts of electricity. The researchers say 1 to 2 megawatt fuel cells could eventually be deployed to power industrial-sized facilities or even small communities.
“Normally, we burn things to create electricity. That means we need oxygen and fuel—or hydrogen,” said fellow WVU research assistant professor Wenyuan Li. “However, fuel cells generate electricity through an electrochemical process that converts the chemical energy from hydrogen and oxygen into electrons by using a catalyst. That’s why we’re developing the perovskite for efficient hydrogen oxidation and oxygen reduction reactions.”
The need for neutrons
Neutrons are an ideal tool for the research team because of the particles’ deep material-penetrating properties and their acute sensitivity to light elements such as lithium. Likewise, the VULCAN diffractometer at SNS is an ideal instrument for studying the three applications the WVU team is investigating. VULCAN has large-area detectors and high penetration capabilities perfect for studying bulky industrial-size samples—such as engine blocks—under an array of simulated operating conditions such as extreme pressures and temperatures.
Using VULCAN, the researchers were able to track in real time the movement of individual elements or atoms in the materials, gaining insights into how the HEOs form during manufacturing to learn whether they formed single or multiple phases during and after the heating and cooling treatments.
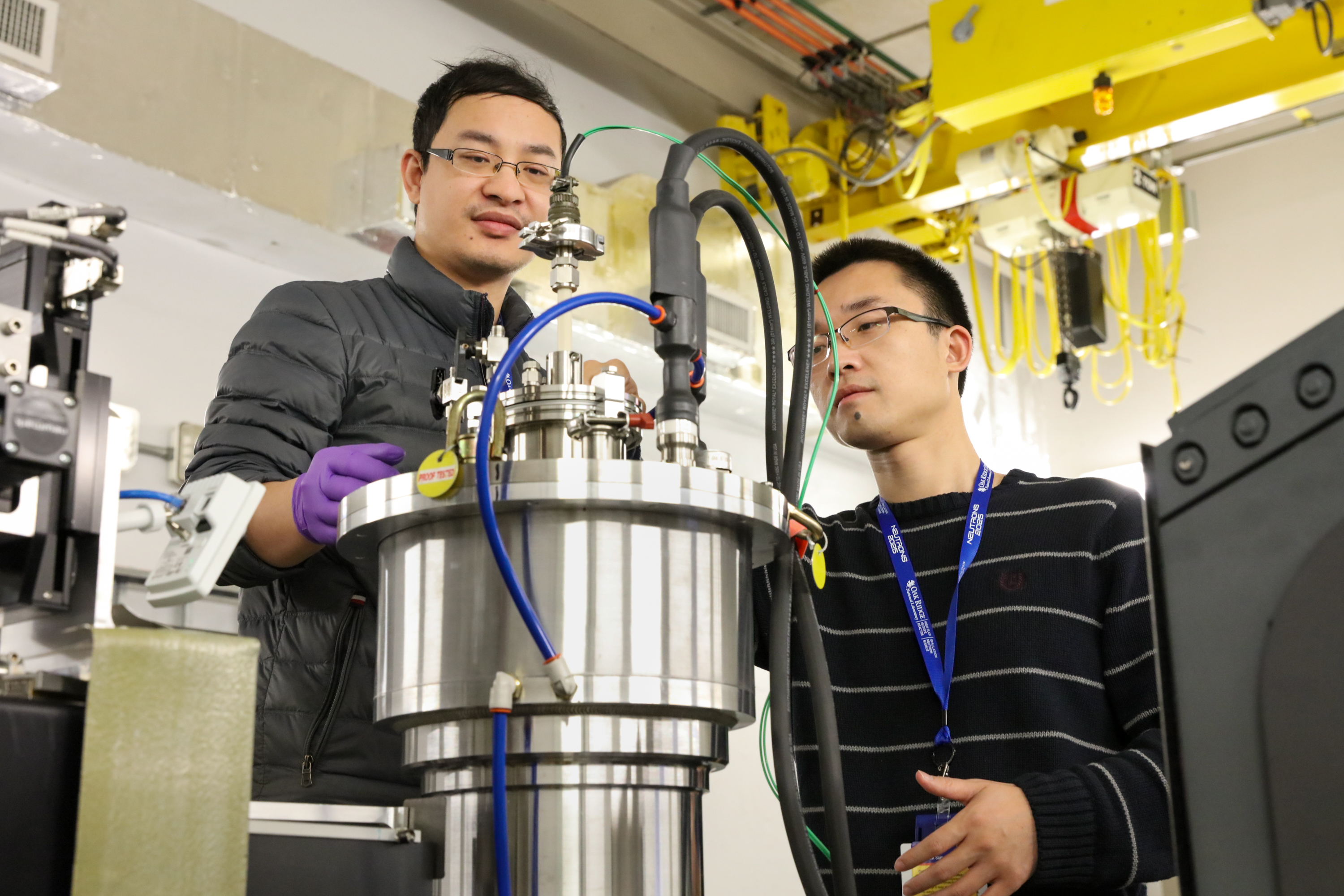
“VULCAN is a very balanced and powerful tool. Some of the in situ measurements we’re doing take between 12 and 20 hours of heating and cooling, and we’re able to monitor how the structures change every minute to 30 seconds,” said Wenyuan Li. “We were able to analyze a lot of materials in a relatively short time.”
The WVU researchers were first-time users of neutron scattering. Data gathered will further aid them in fine-tuning the elemental ratios in their materials and making minute adjustments to their manufacturing methods to ensure materials with the highest quality and efficiency in the end.
SNS is a DOE Office of Science User Facility. UT-Battelle LLC manages ORNL for the DOE Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.—by Jeremy Rumsey